Featured
- Get link
- X
- Other Apps
Calculate Ppm From Concentration
Calculate Ppm From Concentration. For mineral grains (clay, silt and sand sizes), this will typically be 2.65 g/cm3. To convert from ppm by volume to ppm by mass, multiply by the density of the particles.
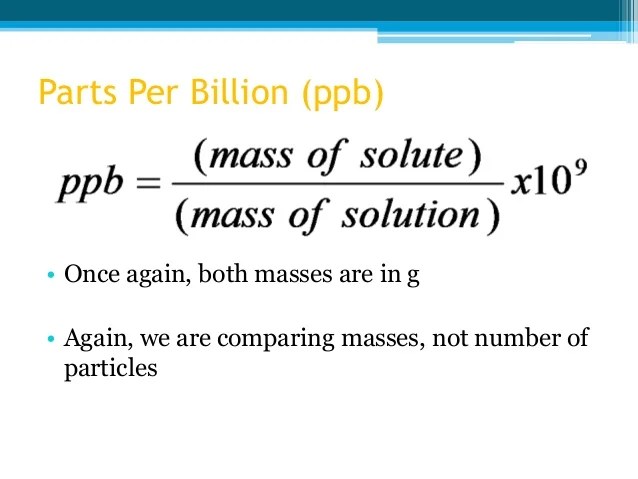
Both ppm (parts per million) and molarity are measures of concentration.did you know that ppm is used in different ways depending on the context? Ppm is often used for low concentration values, typical for air pollution. 1.) select catalog number from dropdown.
To Convert From Ppm By Volume To Ppm By Mass, Multiply By The Density Of The Particles.
We are basicly using parts per million (ppm) in proportion of volume, measured in air. The percentage metrics are all pretty similar in that they describe tiny values of dimensionless variables like the volumetric proportion of no2. How you can calculate just by dividing the mass of the solute by the mass of the solution, then simply multiply by 1,000,000.
The Ppm Calculation Finds The Concentration Of The Solute Ein In The Solution.
We will use the following steps to solve parts per million concentration (ppm) problems: X (ppm) = 10000 ⋅ 1.7% = 17000ppm. Find how many ppm are in 1.7%.
Calculating The Concentration Of A Chemical Solution Is A Basic Skill All Students Of Chemistry Must Develop Early In Their Studies.
Enter values for any three of the four parameters shown below. 1 % = 10⁴ ppm. So the actual concentration of o2 when there is 5 000ppm co2 present (it means 995 000 ppm o2.
By Definition, It Is The Number Of Gas Particles Per Million Air Particles, 1 Ppm = 1/1000000 =.
Percent to ppm conversion table. How to convert moles/liter to ppm. Simple ppm calculator to calculate the concentration of one part per million (ppm) parts of something in water or soil online.
1Ppm = 1Μmole Gas / 1 Mole Air = Vm/M * 1 1Μg Gas /1L Air.
Parts per million is the molar mass,. C (ppm) = 1000000 × msolute / (msolvent + msolute) where msolute = mass of solute, msolvent = mass of solvent. This is a simple tutorial for my regents chemistry students on how to calculate concentrations when using parts per million or ppm
Comments
Post a Comment